 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| Primary adipocyte |
GSM6078547 GSM6078549 GSM6078550
|
|
| hESC |
GSM6078558 GSM6078559 GSM6078560 GSM6078568 GSM6078569 GSM6078571
|
|
|
GSM6078554 GSM6078556 GSM6078557 GSM6078565 GSM6078566 GSM6078567
|
||
|
GSM6078551 GSM6078552 GSM6078553 GSM6078561 GSM6078563 GSM6078564
|
Submission Date: Apr 29, 2022
Summary: Adipocytes are key regulators of human metabolism, and their dysfunction in insulin signaling is central to metabolic diseases including type II diabetes mellitus (T2D). However, the progression of insulin resistance into T2D is still poorly understood. This limited understanding is due, in part, to the dearth of suitable models of insulin signaling in human adipocytes. Traditionally, adipocyte models fail to recapitulate in vivo insulin signaling, possibly due to exposure to supraphysiological nutrient and hormone conditions. We developed a protocol for hPSC-derived adipocytes that uses physiological nutrient conditions to produce a potent insulin response comparable to in vivo adipocytes. After systematic optimization, this protocol allows robust insulin-stimulated glucose uptake and transcriptional insulin response. Furthermore, exposure of sensitized adipocytes to physiological hyperinsulinemia dampens insulin-stimulated glucose uptake and dysregulates insulin-responsive transcription. Overall, our methodology provides a novel platform for the mechanistic study of insulin signaling and resistance using human pluripotent stem cell-derived adipocytes.
GEO Accession ID: GSE201908
PMID: 35714187
Submission Date: Apr 29, 2022
Summary: Adipocytes are key regulators of human metabolism, and their dysfunction in insulin signaling is central to metabolic diseases including type II diabetes mellitus (T2D). However, the progression of insulin resistance into T2D is still poorly understood. This limited understanding is due, in part, to the dearth of suitable models of insulin signaling in human adipocytes. Traditionally, adipocyte models fail to recapitulate in vivo insulin signaling, possibly due to exposure to supraphysiological nutrient and hormone conditions. We developed a protocol for hPSC-derived adipocytes that uses physiological nutrient conditions to produce a potent insulin response comparable to in vivo adipocytes. After systematic optimization, this protocol allows robust insulin-stimulated glucose uptake and transcriptional insulin response. Furthermore, exposure of sensitized adipocytes to physiological hyperinsulinemia dampens insulin-stimulated glucose uptake and dysregulates insulin-responsive transcription. Overall, our methodology provides a novel platform for the mechanistic study of insulin signaling and resistance using human pluripotent stem cell-derived adipocytes.
GEO Accession ID: GSE201908
PMID: 35714187
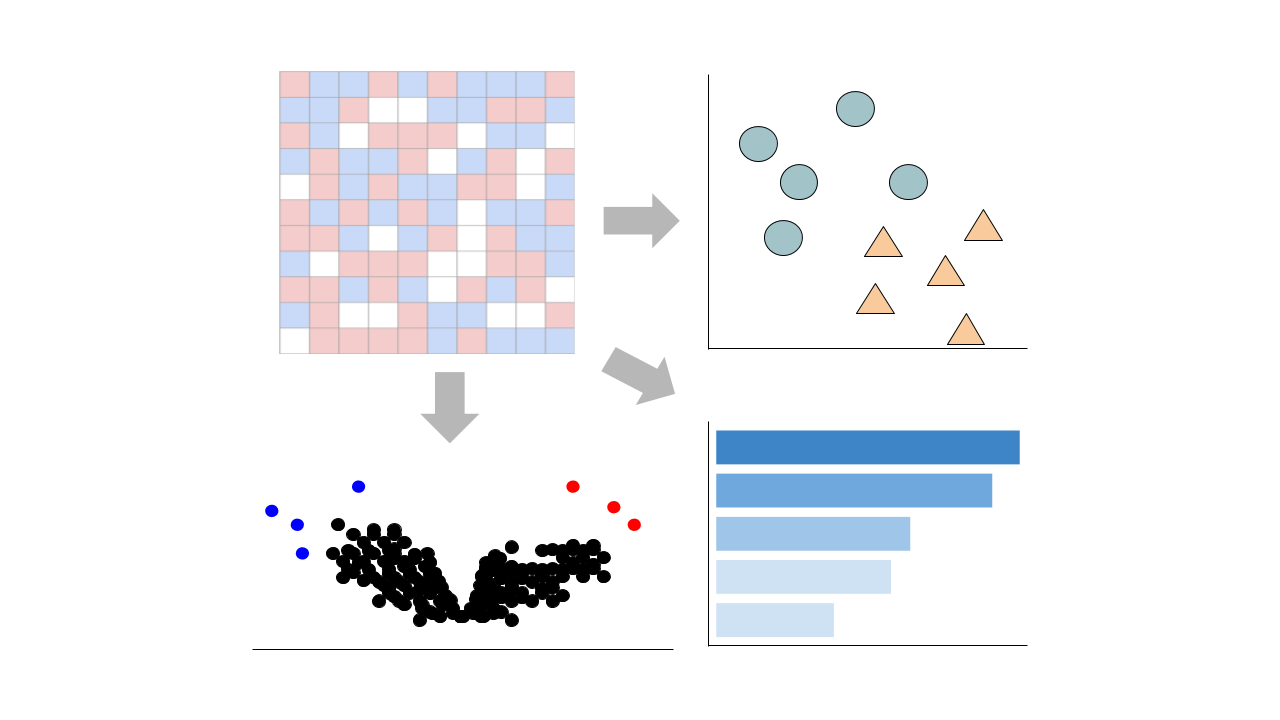
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help