 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| Slc25a47hep+/+ |
GSM5838179 GSM5838180 GSM5838181 GSM5838182
|
|
|
GSM5838171 GSM5838172 GSM5838173 GSM5838174
|
||
| Slc25a47hep-/- |
GSM5838183 GSM5838184 GSM5838185 GSM5838186
|
|
|
GSM5838175 GSM5838176 GSM5838177 GSM5838178
|
Submission Date: Jan 26, 2022
Summary: Background & Aims: Transporters of the SLC25 mitochondrial carrier superfamily bridge cytoplasmic and mitochondrial metabolism by channeling metabolites across mitochondrial membranes and are pivotal for metabolic homeostasis. Despite their physiological relevance as gatekeepers of cellular metabolism, most of the SLC25 family members remain uncharacterized. We undertook a comprehensive tissue distribution analysis of all Slc25 family members across metabolic organs and identified SLC25A47 as a liver-specific mitochondrial carrier. Method: We used a murine loss-of-function (LOF) model to unravel the role of this transporter in mitochondrial and hepatic homeostasis. We performed extensive metabolic phenotyping and molecular characterization of Slc25a47hep-/- mice. Results: Slc25a47hep-/- mice displayed a wide variety of metabolic abnormalities, as a result of sustained energy deficiency in the liver originating from impaired mitochondrial respiration in this organ. This mitochondrial phenotype was associated with a robust activation of the mitochondrial stress response in the liver, which in turn, induced the secretion of several mitokines, amongst which FGF21 played a preponderant role in the translation of the effects of the MSR on systemic physiology. To dissect the FGF21-dependent and -independent physiological changes induced by the loss of Slc25a47, we generated a double Slc25a47-Fgf21 LOF mouse model, and demonstrated that several aspects of the hypermetabolic state was entirely driven by hepatic secretion of FGF21. On the other hand, the metabolic fuel inflexibility induced by loss of Slc25a47 could not be rescued by genetical removal of Fgf21. Conclusion: Collectively, our data place SLC25A47 at the center of mitochondrial homeostasis, which upon dysfunction triggers robust liver-specific and systemic adaptive stress responses.
GEO Accession ID: GSE195479
PMID: 35714811
Submission Date: Jan 26, 2022
Summary: Background & Aims: Transporters of the SLC25 mitochondrial carrier superfamily bridge cytoplasmic and mitochondrial metabolism by channeling metabolites across mitochondrial membranes and are pivotal for metabolic homeostasis. Despite their physiological relevance as gatekeepers of cellular metabolism, most of the SLC25 family members remain uncharacterized. We undertook a comprehensive tissue distribution analysis of all Slc25 family members across metabolic organs and identified SLC25A47 as a liver-specific mitochondrial carrier. Method: We used a murine loss-of-function (LOF) model to unravel the role of this transporter in mitochondrial and hepatic homeostasis. We performed extensive metabolic phenotyping and molecular characterization of Slc25a47hep-/- mice. Results: Slc25a47hep-/- mice displayed a wide variety of metabolic abnormalities, as a result of sustained energy deficiency in the liver originating from impaired mitochondrial respiration in this organ. This mitochondrial phenotype was associated with a robust activation of the mitochondrial stress response in the liver, which in turn, induced the secretion of several mitokines, amongst which FGF21 played a preponderant role in the translation of the effects of the MSR on systemic physiology. To dissect the FGF21-dependent and -independent physiological changes induced by the loss of Slc25a47, we generated a double Slc25a47-Fgf21 LOF mouse model, and demonstrated that several aspects of the hypermetabolic state was entirely driven by hepatic secretion of FGF21. On the other hand, the metabolic fuel inflexibility induced by loss of Slc25a47 could not be rescued by genetical removal of Fgf21. Conclusion: Collectively, our data place SLC25A47 at the center of mitochondrial homeostasis, which upon dysfunction triggers robust liver-specific and systemic adaptive stress responses.
GEO Accession ID: GSE195479
PMID: 35714811
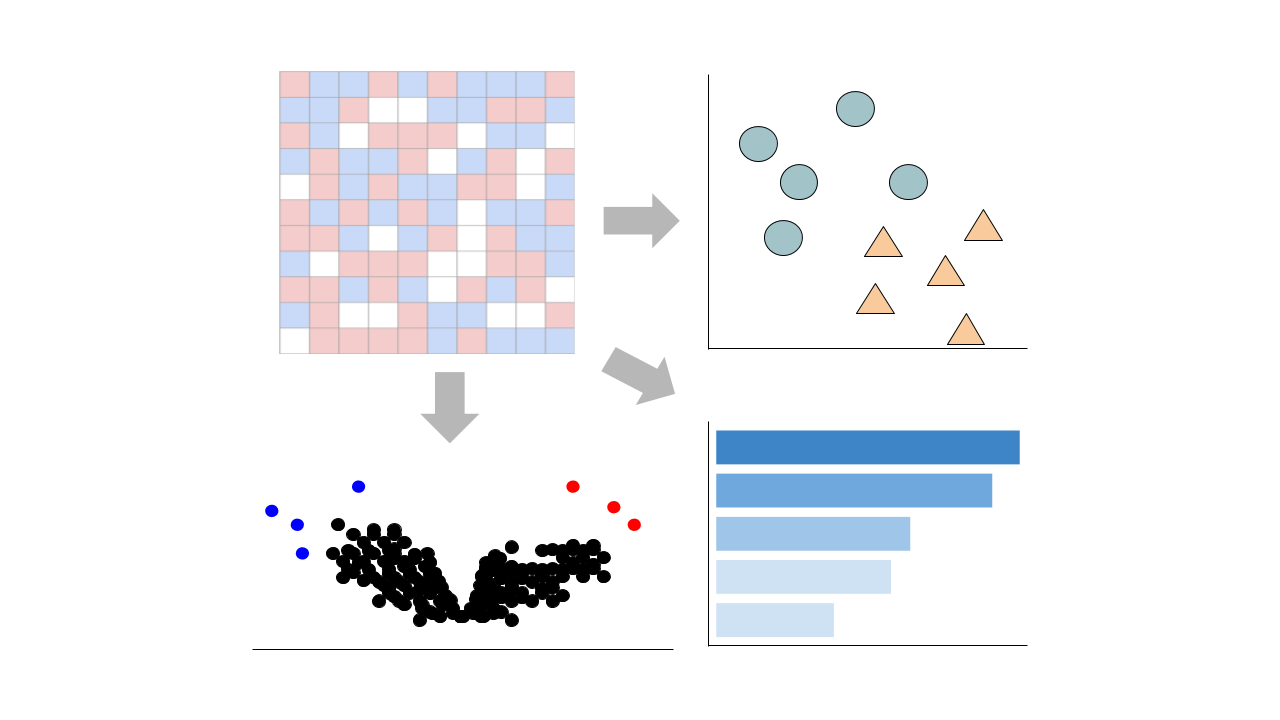
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help