 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| Mouse strain: C57BL/6N |
GSM4636161 GSM4636163 GSM4636165 GSM4636167
|
|
|
GSM4636113 GSM4636115 GSM4636117 GSM4636119
|
Submission Date: Jun 25, 2020
Summary: Purpose: Hyperinsulinemia and insulin resistance are co-existing characteristics of type 2 diabetes, whereas the forerunner initiating the deleterious cycle remains elusive. The temporal transcriptomic landscape of islets (responsible for hyperinsulinemia) and liver (involved in insulin resistance) could provide new insights.
Methods: C57BL/6N mice were fed a 60% high-fat diet (HFD) or control diet (CD) for 24 weeks. RNA-sequencing of islet and liver samples were respectively performed in quadruplicates at six consecutive time points of diet treatments (week 4, 8, 12, 16, 20 and 24), using BGISEQ-500 sequencing platform by the Beijing Genomics Institute (Shenzhen, China).The sequencing raw reads were filtered for low-quality, adaptor-polluted, high content of unknown base reads by SOAPnuke (v1.5.2). We used Trinity (v2.0.6) to perform de novo assembly, and Tgicl (v2.0.6) on cluster transcripts to remove redundancy and get unigenes. The high-quality clean reads were then mapped to the mouse reference genome (GRCm38) via HISAT2 (v2.0.4) and full-length transcriptome database via Bowtie2 (v2.2.5). The gene expression levels were then quantified by RSEM (v1.1.12) and were normalized by the method of fragments per kilobase of exon model per million reads mapped (FPKM). To interpret the functional significance of differentially expressed genes (DEGs), pathway analyses was conducted to determine enriched canonical pathways.
Results: Combined analyses of all 96 samples yielded the identification of 21990 annotated genes. Differentially expressed genes (DEGs) between the two groups (HFD vs. CD) at each time point were identified using the criteria of fold change ≥2 and adjusted P-value ≤0.05. In total, 3844 DEGs were found in islets, of which 33 were shared among all six time points. With regard to liver, 4101 DEGs were discovered throughout 24 weeks of feeding, of which 39 were overlapped. Our islet and liver RNA-sequencing datasets outlined the impact of HFD on dynamics of molecular network at different stages. Correlation analyses of islet and liver modules with metabolic phenotypes illustrated that these two tissues jointly program β-cell adaption to irreversible impairment. Top scored networks combining islet and liver transcriptomes showed potential interactions of genes implicated in cell cycle during week 4, organismal development around week 12, and immune cell trafficking at week 24.
Conclusions: Our data provide a comprehensive landscape of crosstalk between islets and liver in diet-induced diabetes, linking to the development of islet dysfunction and insulin resistance.
GEO Accession ID: GSE153222
PMID: 33817571
Submission Date: Jun 25, 2020
Summary: Purpose: Hyperinsulinemia and insulin resistance are co-existing characteristics of type 2 diabetes, whereas the forerunner initiating the deleterious cycle remains elusive. The temporal transcriptomic landscape of islets (responsible for hyperinsulinemia) and liver (involved in insulin resistance) could provide new insights.
Methods: C57BL/6N mice were fed a 60% high-fat diet (HFD) or control diet (CD) for 24 weeks. RNA-sequencing of islet and liver samples were respectively performed in quadruplicates at six consecutive time points of diet treatments (week 4, 8, 12, 16, 20 and 24), using BGISEQ-500 sequencing platform by the Beijing Genomics Institute (Shenzhen, China).The sequencing raw reads were filtered for low-quality, adaptor-polluted, high content of unknown base reads by SOAPnuke (v1.5.2). We used Trinity (v2.0.6) to perform de novo assembly, and Tgicl (v2.0.6) on cluster transcripts to remove redundancy and get unigenes. The high-quality clean reads were then mapped to the mouse reference genome (GRCm38) via HISAT2 (v2.0.4) and full-length transcriptome database via Bowtie2 (v2.2.5). The gene expression levels were then quantified by RSEM (v1.1.12) and were normalized by the method of fragments per kilobase of exon model per million reads mapped (FPKM). To interpret the functional significance of differentially expressed genes (DEGs), pathway analyses was conducted to determine enriched canonical pathways.
Results: Combined analyses of all 96 samples yielded the identification of 21990 annotated genes. Differentially expressed genes (DEGs) between the two groups (HFD vs. CD) at each time point were identified using the criteria of fold change ≥2 and adjusted P-value ≤0.05. In total, 3844 DEGs were found in islets, of which 33 were shared among all six time points. With regard to liver, 4101 DEGs were discovered throughout 24 weeks of feeding, of which 39 were overlapped. Our islet and liver RNA-sequencing datasets outlined the impact of HFD on dynamics of molecular network at different stages. Correlation analyses of islet and liver modules with metabolic phenotypes illustrated that these two tissues jointly program β-cell adaption to irreversible impairment. Top scored networks combining islet and liver transcriptomes showed potential interactions of genes implicated in cell cycle during week 4, organismal development around week 12, and immune cell trafficking at week 24.
Conclusions: Our data provide a comprehensive landscape of crosstalk between islets and liver in diet-induced diabetes, linking to the development of islet dysfunction and insulin resistance.
GEO Accession ID: GSE153222
PMID: 33817571
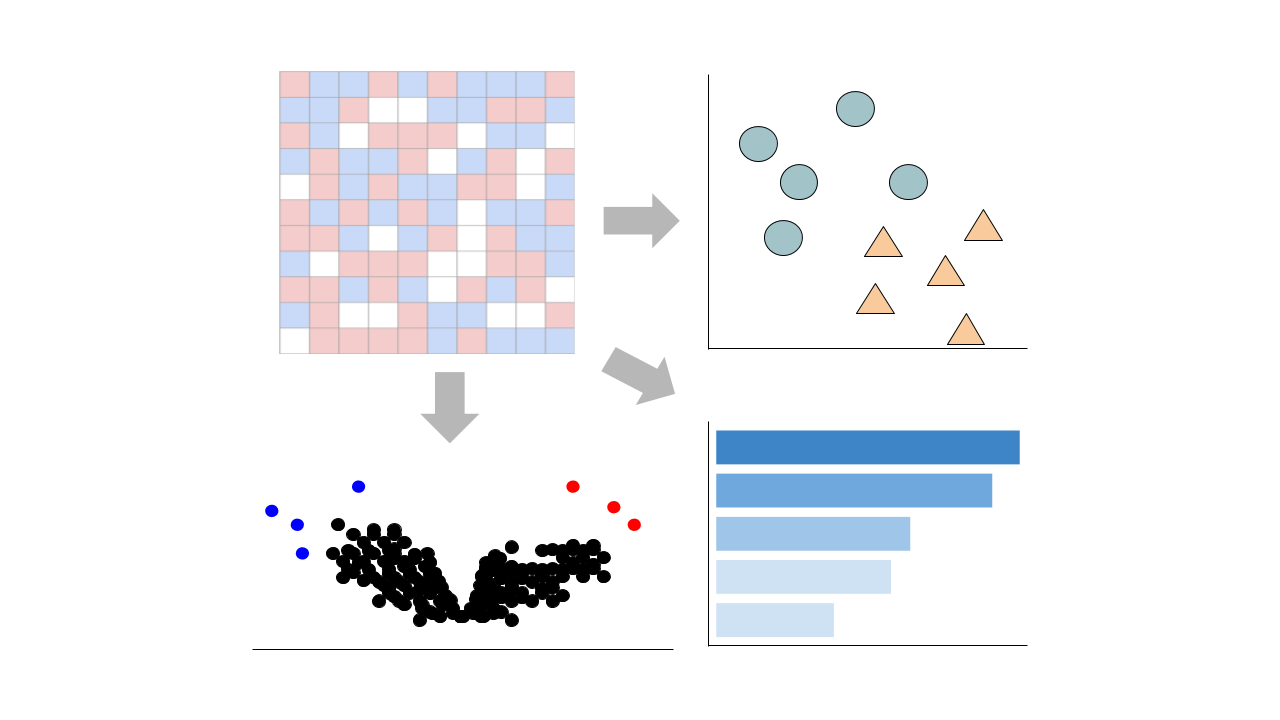
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help