 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| pancreas |
GSM3295679 GSM3295681 GSM3295682 GSM3295684 GSM3295685 GSM3295687 GSM3295715
|
|
|
GSM3295703 GSM3295705 GSM3295706 GSM3295708 GSM3295709 GSM3295711
|
||
|
GSM3295689 GSM3295690 GSM3295692 GSM3295693 GSM3295694 GSM3295717 GSM3295718
|
||
|
GSM3295713 GSM3295714 GSM3295725 GSM3295727 GSM3295728
|
||
|
GSM3295723 GSM3295724
|
||
|
GSM3295672 GSM3295673 GSM3295675 GSM3295676 GSM3295678
|
||
|
GSM3295721
|
||
|
GSM3295720
|
||
|
GSM3295696 GSM3295697 GSM3295699 GSM3295700 GSM3295702
|
Submission Date: Jul 20, 2018
Summary: Natural and stable cell identity switches, where terminally-differentiated cells convert into different cell-types when stressed, represent a widespread regenerative strategy in animals, yet they are poorly documented in mammals. In mice, some glucagon-producing pancreatic α-cells become insulin expressers upon ablation of insulin-secreting β-cells, promoting diabetes recovery. Whether human islets also display this plasticity for reconstituting β-like cells, especially in diabetic conditions, remains unknown. Here we show that two different islet non-β-cell types, α- and γ–cells, obtained from deceased non-diabetic or diabetic human donors can be lineage-traced and induced to produce insulin and secrete it in response to glucose. When transplanted into diabetic mice, converted human α-cells reverse diabetes and remain producing insulin even after 6 months. Insulin-producing α-cells maintain α-cell markers, as seen by deep transcriptomic and proteomic characterization, and display hypo-immunogenic features when exposed to T-cells derived from diabetic patients. These observations provide conceptual evidence and a molecular framework for a mechanistic understanding of in situ cell plasticity in islet cells, as well as in other organs, as a therapy for degenerative diseases by fostering the highly-regulated intrinsic cell regeneration.
GEO Accession ID: GSE117454
PMID: 30760930
Submission Date: Jul 20, 2018
Summary: Natural and stable cell identity switches, where terminally-differentiated cells convert into different cell-types when stressed, represent a widespread regenerative strategy in animals, yet they are poorly documented in mammals. In mice, some glucagon-producing pancreatic α-cells become insulin expressers upon ablation of insulin-secreting β-cells, promoting diabetes recovery. Whether human islets also display this plasticity for reconstituting β-like cells, especially in diabetic conditions, remains unknown. Here we show that two different islet non-β-cell types, α- and γ–cells, obtained from deceased non-diabetic or diabetic human donors can be lineage-traced and induced to produce insulin and secrete it in response to glucose. When transplanted into diabetic mice, converted human α-cells reverse diabetes and remain producing insulin even after 6 months. Insulin-producing α-cells maintain α-cell markers, as seen by deep transcriptomic and proteomic characterization, and display hypo-immunogenic features when exposed to T-cells derived from diabetic patients. These observations provide conceptual evidence and a molecular framework for a mechanistic understanding of in situ cell plasticity in islet cells, as well as in other organs, as a therapy for degenerative diseases by fostering the highly-regulated intrinsic cell regeneration.
GEO Accession ID: GSE117454
PMID: 30760930
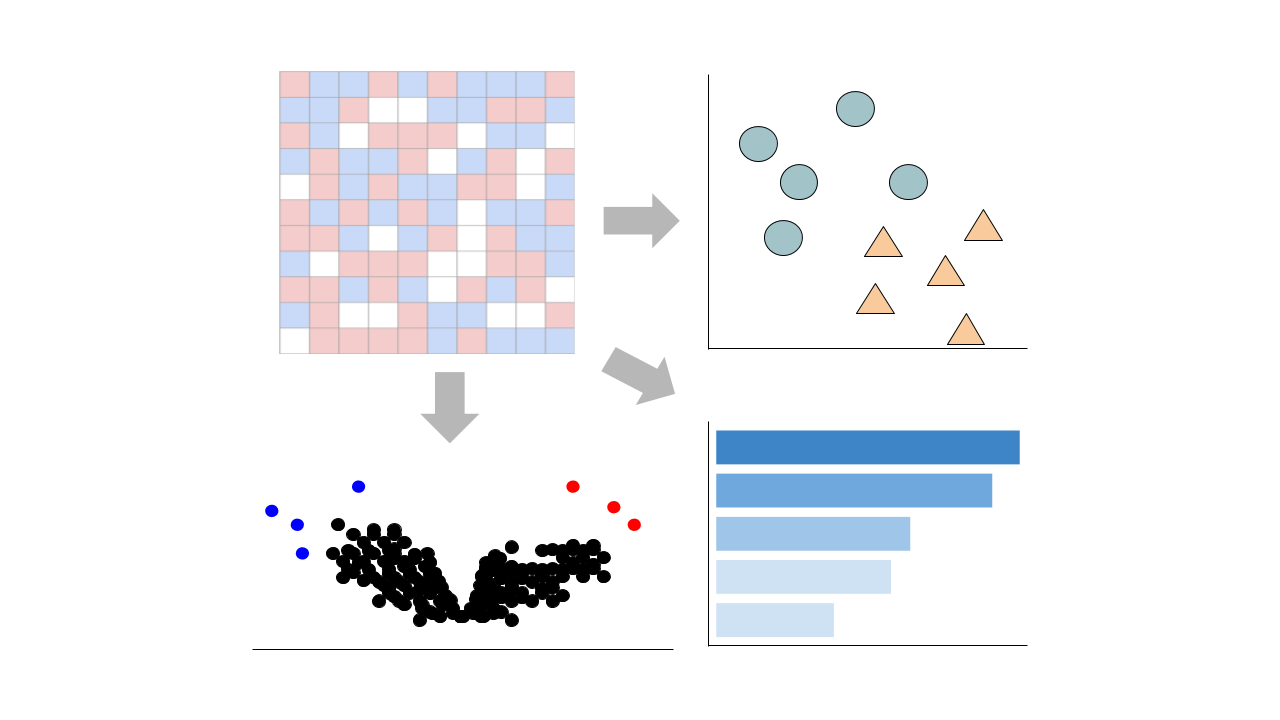
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help