 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| Adipocytes |
GSM3416384 GSM3416385 GSM3416386
|
|
|
GSM3416387 GSM3416388 GSM3416389
|
||
|
GSM3416393 GSM3416394 GSM3416395
|
||
|
GSM3416390 GSM3416391 GSM3416392
|
||
|
GSM3177920 GSM3177921 GSM3177922 GSM3177926 GSM3177927 GSM3177928 GSM3177932 GSM3177933 GSM3177934 GSM3177938 GSM3177939 GSM3177940 GSM3177944 GSM3177945 GSM3177946
|
||
|
GSM3177923 GSM3177924 GSM3177925 GSM3177929 GSM3177930 GSM3177931 GSM3177935 GSM3177936 GSM3177937 GSM3177941 GSM3177942 GSM3177943 GSM3177947 GSM3177948 GSM3177949
|
Submission Date: Jun 06, 2018
Summary: Thiazolidinedione drugs (TZDs) target the transcriptional activity of PPARg to reverse insulin resistance in type 2 diabetes, but side effects limit their clinical use. Here, using human adipose stem cell-derived adipocytes, we demonstrate that single-nucleotide polymorphisms (SNPs) were enriched at sites of patient-specific PPARg binding, which correlated with the individual-specific effects of TZD rosiglitazone (rosi) on gene expression. Rosi induction of ABCA1, which regulates cholesterol metabolism, was dependent upon SNP rs4743771, which modulated PPARg binding by influencing the genomic occupancy of its cooperating factor NFIA. Conversion of rs4743771 from the inactive SNP allele to the active one by CRISPR/Cas9-mediated editing rescued PPARg binding as well as rosi-induction of ABCA1 expression. Moreover, rs4743771 is a major determinant of undesired serum cholesterol increases in rosi-treated diabetics. These data highlight human genetic variation that impacts PPARg genomic occupancy and patient responses to antidiabetic drugs, with implications for developing personalized therapies for metabolic disorders
GEO Accession ID: GSE115421
PMID: 30639037
Submission Date: Jun 06, 2018
Summary: Thiazolidinedione drugs (TZDs) target the transcriptional activity of PPARg to reverse insulin resistance in type 2 diabetes, but side effects limit their clinical use. Here, using human adipose stem cell-derived adipocytes, we demonstrate that single-nucleotide polymorphisms (SNPs) were enriched at sites of patient-specific PPARg binding, which correlated with the individual-specific effects of TZD rosiglitazone (rosi) on gene expression. Rosi induction of ABCA1, which regulates cholesterol metabolism, was dependent upon SNP rs4743771, which modulated PPARg binding by influencing the genomic occupancy of its cooperating factor NFIA. Conversion of rs4743771 from the inactive SNP allele to the active one by CRISPR/Cas9-mediated editing rescued PPARg binding as well as rosi-induction of ABCA1 expression. Moreover, rs4743771 is a major determinant of undesired serum cholesterol increases in rosi-treated diabetics. These data highlight human genetic variation that impacts PPARg genomic occupancy and patient responses to antidiabetic drugs, with implications for developing personalized therapies for metabolic disorders
GEO Accession ID: GSE115421
PMID: 30639037
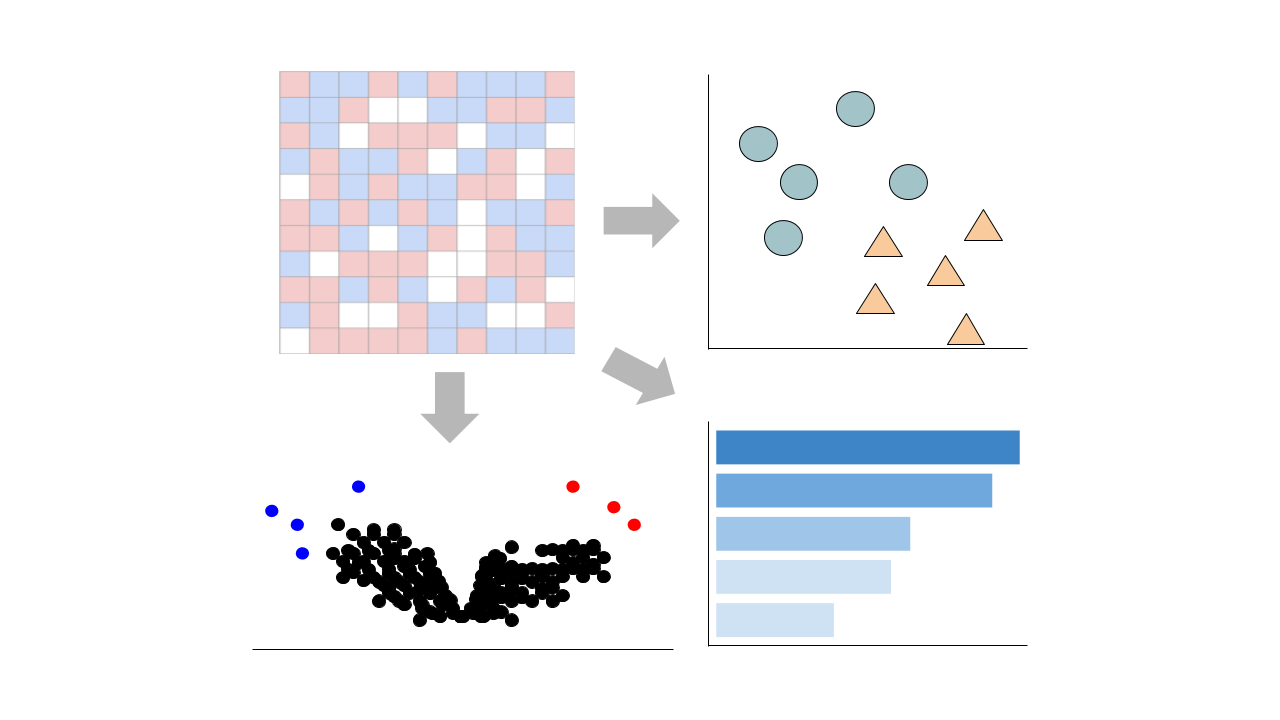
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help