 Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Gene counts are sourced from ARCHS4, which provides uniform alignment of GEO samples.
You can learn more about ARCHS4 and its pipeline here.
Select conditions below to toggle them from the plot:
| GROUP | CONDITION | SAMPLES |
|---|---|---|
| islets of Langerhans |
GSM2735601
|
|
|
GSM2735597 GSM2735598 GSM2735599
|
||
|
GSM2735600
|
Submission Date: Aug 08, 2017
Summary: Our current understanding of the pathogenesis of T1D arose in large part from studies using the non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D). Of concern, therapeutic interventions shown to significantly dampen or even reverse disease in mouse models have not successfully translated into interventions in human T1D. The present study addresses this disconnect in research translation by directly analyzing human donor islets from individuals with T1D, aiming to provide insight into disease mechanisms and identify potential target pathways for therapeutic intervention. We obtained human islets from a young individual with short-duration T1D, an older individual with long-duration T1D and three non-diabetic donors, and performed unbiased functional genomic analysis by high depth RNA sequencing on these unique cases. This analysis identified several inflammatory pathways upregulated in short-duration disease, which surprisingly included many components of innate immunity. Subsequent manipulation of one of these pathways/factors in NOD mice resulted in a significant reduction in diabetes progression when inhibition occurred early in disease progression. Taken together our data demonstrates that the direct analysis of human islets is essential for identifying relevant and promising novel targets for translation into effective therapeutic interventions for human T1D.
GEO Accession ID: GSE102371
PMID: 29569324
Submission Date: Aug 08, 2017
Summary: Our current understanding of the pathogenesis of T1D arose in large part from studies using the non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D). Of concern, therapeutic interventions shown to significantly dampen or even reverse disease in mouse models have not successfully translated into interventions in human T1D. The present study addresses this disconnect in research translation by directly analyzing human donor islets from individuals with T1D, aiming to provide insight into disease mechanisms and identify potential target pathways for therapeutic intervention. We obtained human islets from a young individual with short-duration T1D, an older individual with long-duration T1D and three non-diabetic donors, and performed unbiased functional genomic analysis by high depth RNA sequencing on these unique cases. This analysis identified several inflammatory pathways upregulated in short-duration disease, which surprisingly included many components of innate immunity. Subsequent manipulation of one of these pathways/factors in NOD mice resulted in a significant reduction in diabetes progression when inhibition occurred early in disease progression. Taken together our data demonstrates that the direct analysis of human islets is essential for identifying relevant and promising novel targets for translation into effective therapeutic interventions for human T1D.
GEO Accession ID: GSE102371
PMID: 29569324
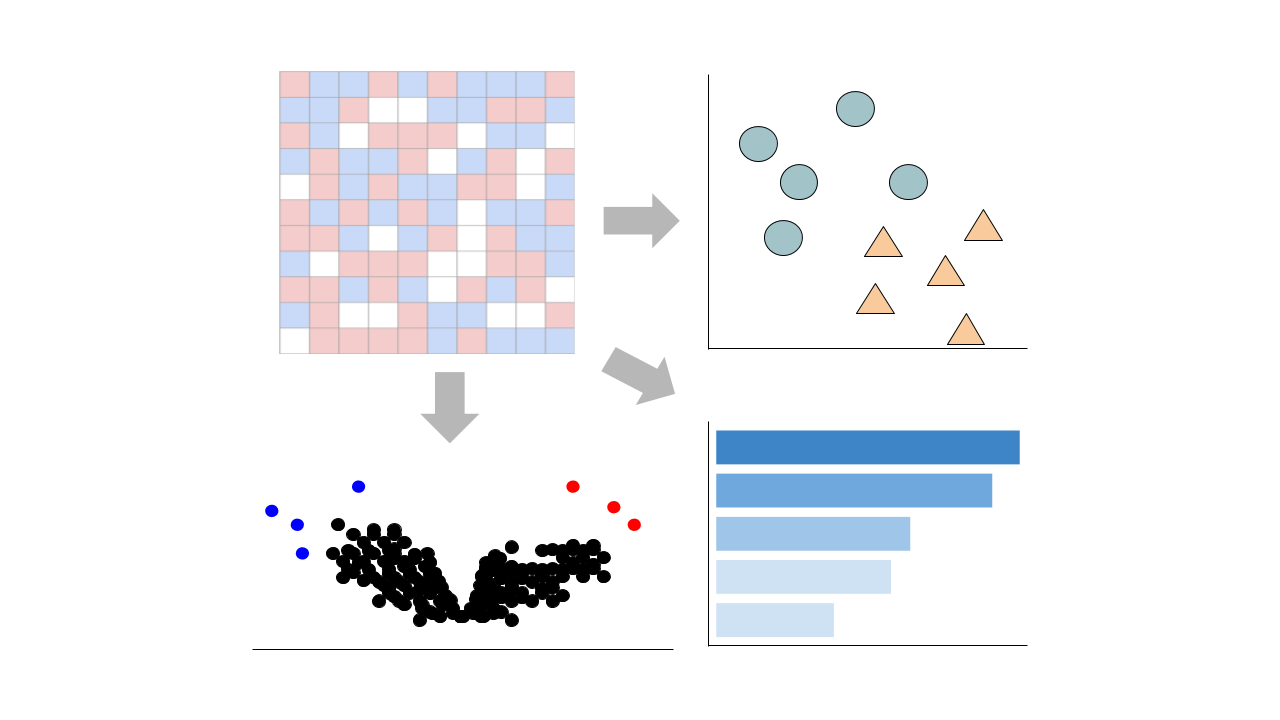
Visualize Samples
 Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Visualizations are precomputed using the Python package scanpy on the top 5000 most variable genes.
Precomputed Differential Gene Expression
 Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Differential expression signatures are automatically computed using the limma R package.
More options for differential expression are available to compute below.
Signatures:
No precomputed signatures are currently available for this study. You can compute differential gene expression on the fly below:
Select conditions:
Control Condition
Perturbation Condition
Only conditions with at least 1 replicate are available to select
 Differential expression signatures can be computed using DESeq2 or characteristic direction.
Differential expression signatures can be computed using DESeq2 or characteristic direction.
This pipeline enables you to analyze and visualize your bulk RNA sequencing datasets with an array of downstream analysis and visualization tools. The pipeline includes: PCA analysis, Clustergrammer interactive heatmap, library size analysis, differential gene expression analysis, enrichment analysis, and L1000 small molecule search.
 Chatbot
Chatbot Single Gene Queries
Single Gene Queries
 Gene Set Queries
Gene Set Queries
 Bulk Studies
Bulk Studies
 Single Cell Studies
Single Cell Studies
 Hypotheses
Hypotheses
 Resources
Resources
 Contribute
Contribute
 Downloads
Downloads About
About
 Help
Help